
How To Extract Silver From Silver Acetate
In our life, many people like to buy some silver or some silver jewelry, because in many occasions, these things will be more practical, for example, we can use it to make some crafts or used as some ornaments. Of course, many people will choose to extract some silver from some old silver to use. Today we are going to talk about extracting silver from silver acetate. What should we do?
The purification process of silver acetate recovery is mainly the reaction of silver and acetic acid solution, the impurity in the solution is removed by filtration, and then sodium hydroxide is added for washing, and then titrated with dilute nitric acid and sulfuric acid, so as to achieve the purpose of purification. In this process, it should be noted that glass rods or coarse filtering devices are required. Secondly, it is necessary to control the concentration and temperature of nitric acid and sulfuric acid solution during the experiment.
1. Add an appropriate amount of silver acetate and a small amount of water to a 100ml beaker and stir well.
2. Pour the solution in the beaker into the beaker with copper wire, then put the beaker with copper wire into the large beaker with silver acetate and water, pour the solution in the beaker into the beaker with alcohol, and stir well.
3. Then, use an eyedropper to drop the alcohol into the beaker containing the silver powder and let it dissolve thoroughly, stirring constantly with a glass rod until the silver powder is completely dissolved.
4. Finally, remove all the dissolved silver acetate solution from the solution with a glass rod for precipitation. Finally, the precipitated silver powder is smelted to obtain high purity silver.
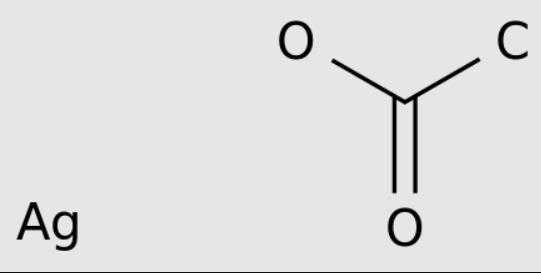
Silver acetate is a coordination compound with the empirical formula CH3CO2Ag (or AgC2H3O2). It is a light-sensitive white crystalline solid that is a useful reagent in the laboratory and is a source of silver ions lacking oxidizing anions.
Author Bio
Article Comments
No Comments!
At present there are zero comments on this article.
Why not be the first to make a comment?
Article Categories
There are zero sub-categories in this parent category.
There are zero sub-categories in this parent category.











